

Their reactivity increases as we go down. The trend is that from top to bottom in the alkali metals group, the reactivity levels increase.

So, large group I metal atoms are the most reactive and large halogens are the least reactive. The most reactive metals are found on the left of the periodic table, in the blue column, known as the alkali metals. His O 2 saturation does not improve and remains at 88 with oxygen at 6 L/min. The solubility of the hydroxides increase down the group causing the. In one of the severity groups the albuterol group had a shorter time of. Group I metals want to lose an electron while halogen atoms want to gain an electron. strontium and barium react with cold water and the reactivity increases from. The small atoms are at the top of the groups. This is the opposite trend to that seen in the alkali metals in Group 1 of the periodic. This is best when the atom is as small as possible and so fluorine is the most reactive of the halogens.īreak the idea down into clar statements:Īttraction between nucleus and electron is greatest for small atoms. Each time, lithium is the least reactive of the three elements, and potassium the most: Reactivity increases as you go down Group I. The non-metal elements in Group 7 - known as the halogens - get less reactive as you go down the group. This extra electron is held tightest when it is placed in a shell close to the nucleus. The halogens react by gaining an electron to form the halide ion (eg chlorine becomes chloride). The biggest atoms are at the bottom of the group so they are the most reactive. As you move down the group, the amount of electron shielding increases, meaning that the electron is less attracted to the nucleus. HF is a weak acid due to its high bond energy. This is because group 7 elements react by gaining an electron. Hence, the acid strength increases down the group as bond energies decrease. This can be explained by the increase in ease at losing two outer electrons as we descend the. HX (g) + (aq) H +(aq) + X -(aq) The acid strength depends upon the ease at which the HX bond can be broken. Reactivity of Group II elements increases down the group. Their solutions are acidic due to the formation of H + ions. This can happen easiest if the electron is in a shell that is a long ay from the nucleus so that there is less attraction between the nucleus and the electron. All the hydrogen halides are very soluble in water. Why does reactivity increase down group 2 The second ionisation energies - which make up most of the energy of the reaction - decreases down group 2 due to increasing atomic radius and electron shielding. Group I metals are aiming to lose an electron from their outside shell.
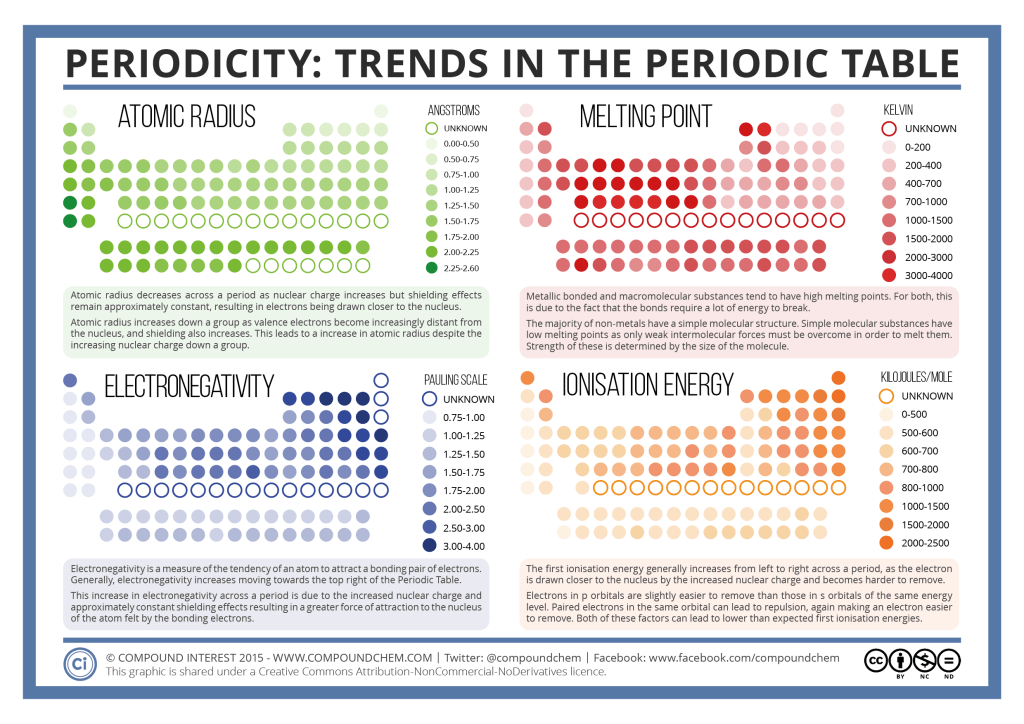
The answer lies in understanding what the atoms are trying to do. I get why it does in group 1, but I don't get why it does in group 7? This is because group 7 elements react by gaining an electron. Except for the beryllium and magnesium chloride, the chlorides of other members of the group 2 imparts characteristic color to the flame.Why do the reactivity of the group 1 atoms increase as you go down the group, but the reactivity of group 7 decreases as you go down the group. The alkali metals readily react with halogens to form ionic halides of the form $M^O.$ This tendency of forming halide hydrates gradually decreases down the group from Mg to Ba. Non-metals Period - reactivity increases as you go from the left to the right across a period. That is why as you go up a group Chemical Reactivity increases because it is easier for elements to gain electrons when they have high electronegativity. These elements are called alkali metals because they readily dissolve in water to form hydroxides which are strongly alkaline in nature. Group - reactivity increases as you go down a group The farther to the left and down the periodic chart you go, the easier it is for electrons to be given or taken away, resulting in higher reactivity. Chemical Reactivity decrease as you go down the group For Non-Metals, the farther right-up in the table you go, the higher the electronegativity. They constitute the six elements namely, lithium(Li), sodium(Na), potassium(K), rubidium(Rb), cesium(Cs) and francium(Fr). Reactivity, unlike other periodic trends, does not increase or decrease going across a period or up and down a group. The elements belonging to group 1 are called alkali metals.


 0 kommentar(er)
0 kommentar(er)
